By BILL BRANDON

Bill Brandon is an architect by training. Since 1972, his design/build firm has been engaged in substantial residential additions and renovations in the DC metro area. His designs have incorporated aspects of building energy efficiency including early adapter status of passive solar, super insulation, underground structures and ground source heat pumps. He has also been engaged in furniture manufacturing, starting a molded plywood furniture business in Belize and it was there that he first became involved in bio-based products and bioenergy. He has since become deeply engaged with the biofuels and materials industry. He also has a deep understanding of engines and alternative fuels. His articles have been published in Biofuels USA and the Biofuels Digest. His present focus is on technology integration and deployment, urban and farm wastes to energy and vertical or City Farming.
Executive Summary
This two-part White Paper (read part two here) presents the proposition that we need to transition away from the legacy fuels we use today to modern liquid and gas fuels[1] that: 1) reduce or eliminate polycyclic aromatic hydrocarbons (PAHs, polyaromatic hydrocarbons) also known as ultrafine particles that are significantly detrimental to our health; 2) reduce greenhouse gas intensity; and 3) allow auto manufacturers to produce vehicles with high engine efficiencies and thereby lowering driving costs.
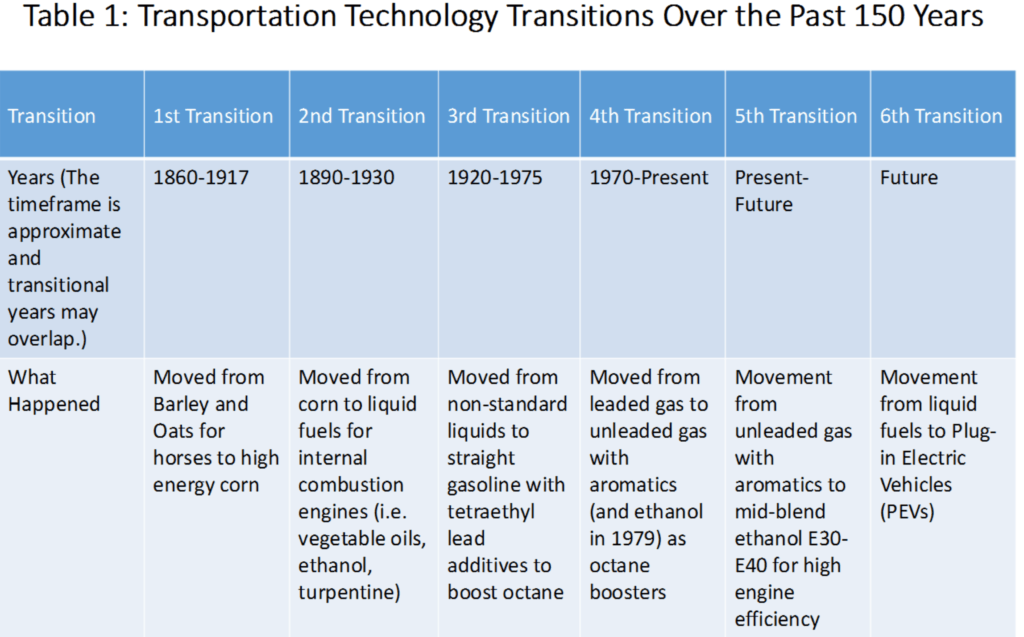
A historical background is presented from our ‘original fuel’ of barley and oats fed to horses and mules through creation of the Environmental Protection Agency (EPA) and continuing modifications and rules originating from the Clean Air Act of 1970.[2] Technical, economic and political aspects of over 150 years of our transportation transitions are summarized to give background for the transitions we need to embark on today.
[1] The author uses the term “modern” instead of “advanced” in this article as ‘Advanced fuel’ has a definition within the renewable fuel standard. His introduction of the word ‘Modern’ is to differentiate between that definition and what is really needed from a fuel standpoint that is agnostic to renewable vs fossil.
[2] The EPA was formed in 1970 and the Clean Air Act was passed in 1970 as part of its enabling legislation. The clean fuels program was finalized in 1973. The three year interim was taken up with discussions and industry input on how the clean fuels program was to be constructed.
That transition is one of moving to ‘Carbon Light’ fuels that consist of dimethyl ether (DME); E30 – E40 (30 or 40% ethanol blend with gasoline); and the remainder being gasoline without aromatics, natural or bio methane and hydrogen that can be either fossil sourced or renewably sourced. By selecting these specific fuel formulations, we can improve emission profiles to lower existing health risks, reduce greenhouse gas (GHG) emissions and improve fuel economy. In recognition of the near-term need to partially rely on fossil resources; this transition focuses on fuel production better matched to our oil, gas and renewable resources in the US. It allows for full competition of renewable fuels or blending components within all fuel markets and paves a way to retire fossil fuels without economic disruption when peak price oil or gas arrives or measures are enacted to restrict GHGs.
Transition #1- Moving from Barley and Oats for Draft Animal Feed to Corn
Our country has seen many transitions, both large and small, in travel technology and fuels. Trains replaced wagons, coaches and canals, but for local travel, personal and farm use, horses and mules remained our early technologies. Small grains (barley and oats) were a significant fuel source for these animals. With westward development a better energy fuel was produced in the 1860’s by James L. Reid, whose yellow dent corn provided superior production per acre over barley or oats. Because of its high starch content and its high rate of production, corn became the major source of energy for our draft animals, reaching 111 million acres in 1917. The use of corn as a significant substitute for pasture, barley and oats was a major transformation in our transportation fuel supply and is a major reason we still grow so much of this same dent corn today. Yet, the American farmer was going to lose its major cash market to petroleum. This gradual slip became a major oversupply problem after World War I (WWI) and as the rest of the country ‘roared’ in the ‘20s’, the rural and farm economy was in steep decline only to be made worse by the great depression.
Between 1830 and 1860, ethanol, primarily made from corn in the United States, was the primary source for ‘lamp oil’. In 1860, German inventor, Nicolaus Otto, used ethanol to fuel his internal combustion engines and was later favored by Henry Ford. By 1860, John D. Rockefeller had developed petroleum distillation to separate lubricating oil and kerosene as a lamp oil, leaving the components with higher volatility as a waste we now call gasoline. In 1862, Congress passed an alcohol tax to help retire Civil War debt. This essentially wiped out the U.S. industrial ethanol industry, leaving Rockefeller an open market for kerosene.
Transition #2 – From Grain to Liquid Fuels
Another transportation transformation was on the way in the form on the internal combustion engine (IC engine). Some saw this change coming and made the transition. First were the reluctant Studebaker brothers who were influenced by their brother-in-law, Fredrick Fish. The Studebaker Manufacturing Co was founded in 1852 in South Bend, Indiana. Blacksmiths by trade, they made sturdy wagons selling to the U.S. army from the Civil War through WWI and selling a valued Conestoga wagon from their St. Louis sales office to pioneers heading west. They began dabbling with supplying auto parts in 1896 and built their first electric vehicle in 1902. They introduced gasoline-powered vehicles in 1904, which became their main-selling models. Studebaker made their last wagon in 1920.
William Durant, founder of General Motors, saw the change coming and adapted. Durant gave up the profitable Durant-Dort Carriage Company, which in 1890 was the largest manufacturer of carriages in the United States, to embrace automobiles. Starting with Buick in 1904, by 1909 through United Motors (to be later named General Motors) Durant had bought and consolidated an additional 8 auto manufacturers and numerous parts suppliers. The early familiar names were Buick, Olds and Cadillac. (Ironically, Henry Ford failed twice before succeeding with the Ford Motor Company. His second attempt was sold to his investors because of his slow pace developing his production process. They wanted a vehicle to show cash flow. This company became Cadillac.) Some production assets of Durant-Dort were stranded, but the marketing and supply chain knowledge from the carriage company transferred. Durant sold a variety of carriages from simple to fancy. This marketing strategy was different from Ford and Studebaker and became what General Motors (GM) was known for, a series of brands from the entry Chevrolet to the luxury Cadillac.
In 1906, the Civil War era ethanol tax was drastically reduced and, for a short time, ethanol was competitive with gasoline until light crude from the east Texas Humble oil fields were developed. In 1912, gasoline sales surpassed kerosene sales and the top auto makers were 1) Ford, 2) GM and 3) Studebaker. There was also a growing realization that a better fuel with higher octane was needed.
The Dayton, Ohio Connection – Discovery of Lead as an Octane Booster in Gasoline
Dayton, Ohio, home of the Wright Brothers, became an interesting location for both significant advances and failures in the automobile and fuel story. A very smart and charming deal-maker by the name of (Col.) Edward Deeds was an employee of John Patterson (the second name in Wright-Patterson Air Force Base) at the National Cash Register Company. Deeds was a supporter of the Wright Brothers and helped them get off the ground so to speak. His interest was in electrical engineering but lacked advanced training. He had the idea of electrifying the cash register, but felt he needed more help. He found a young, bright electrical engineer by the name of Charles Kettering and together they electrified the cash register and sent ‘National Cash’ into dominance in the cash register field. This collaboration led them to form the Dayton Engineering Lab Company (DELCO) in 1909. DELCO became a well-known auto parts supplier for many years. Delco solved two major problems with gasoline powered vehicles. First was a high voltage ignition system which prevented stalling problems and second was the electric self-starter. Most of DELCO’s contributions to the auto industry were around electrical and combustion issues. A young DELCO employee, Thomas Midgley, discovered that adding lead to gasoline retarded self-ignition and served as an octane booster in gasoline. Charles Kettering and Henry Ford argued for using ethanol as an octane enhancer and this discovery was put on the back bench. Other events were to come into play, however.
Fellow Daytonian, Orville Wright, co-inventor of the airplane, died of typhoid in 1912. The Wright brothers, Orville and Wilbur and their sister, Katharine, were very close. Orville’s death devastated Wilbur and Katharine. Wilbur sold the assets of the Wright Aeroplane Company to a group of New York investors including Glenn Curtis, his main competitor. While Wright sold the hard assets, government contracts and licenses, he kept the patents. Deeds, who had helped the Wright brothers, felt this was either a bad move or an opportunity. We have no record of what may have transpired between Wright and Deeds. In late 1916 Deeds and Kettering sold DELCO to Durant and GM and in early 1917 started the Dayton-Wright Company. They promised Wilbur they would defend all of his patent infringement suits. With Wilbur Wright as a silent partner, they gained control of all of Wright’s patents. The Dayton-Wright Company made almost 3400 airplanes for the US Army during WWI.
GM Asserts Dominance Through the Industrial Elite
The history of GM is somewhat contorted with Durant building the company, being kicked out by investors, developing Chevrolet with new investors, regaining control of GM through Chevrolet and being kicked out again. With GM’s second takeover by Durant, Chevrolet and GM were highly backed by Pierre S. DuPont. With GM’s purchase of DELCO they now had basic data for tetra-ethyl lead. DELCO was now GM’s research branch still located in Moraine, Ohio outside of Dayton with Charles Kettering as head of research. Kettering did not initially favor using lead as an octane enhancer but believed that oil was a limited resource and that ethanol would become the ‘fuel of the future’. He apparently had Durant’s support. Durant was kicked out as CEO in 1920 as he and Kettering appeared to be involved in an internal dispute. Subsequently GM (owner or the use patent for lead) and Standard Oil of New Jersey (ESSO and owner of the manufacturing patent for tetraethyl lead) formed the Ethyl Corporation with a contract with DuPont to manufacturer this lead additive.
Transition #3 – Introduction of Tetraethyl Lead into Gasoline
ESSO began introducing tetraethyl lead into gasoline in 1923 but halted production in late 1924 after the deaths of several factory workers. Government hearings were held regarding lead’s health issues, but it was deemed safe and no action was taken although the Ethyl Corporation agreed to limit lead to 3cc per gallon. Again, we have no record of exactly what happened and corporate archives are missing significant documents, but events speak for themselves. Lead was a cheap additive, and while ethanol could compete with lead as an antiknock agent it would take sales volume from oil companies and could not develop royalty payments. This was the start of the third transformation for transportation fuels. Kettering remained with GM until his retirement in 1947. In 1927 he formed the C. F. Kettering Foundation for the Study of Chlorophyll and Photosynthesis at Antioch College (Yellow Springs, Ohio) indicating his continuing interest in renewable fuels. Thomas Midgley took a prolonged rest in Florida starting in 1923 to recover from lead poisoning.
In 1920, Progressive Politics Loses Out to Incumbent Money Interests
A greater political historical view puts some of these occurrences into perspective. The industrial elite largely controlled the late 19th century politics. Working conditions were poor and labor unrest was prevalent. Ohio was an intersection of growing industrialization and rural agriculture. After the Civil War, presidents oscillated back and forth between the northeast (New York and New Jersey) and the near Midwest (Ohio, Indiana and Illinois). The political stalemate of the post-Civil War era gave way to the Republican-dominated Fourth Party System, which began with the Progressive Era. The Sherman Anti-Trust Act had been passed under President Benjamin Harrison. William McKinley was a moderate Republican Governor of Ohio and was selected by the industrial elite as their best bet against William Jennings Bryan, the populist Democrat from Nebraska, while pushing NY Governor Theodore Roosevelt to the second position as VP. With McKinley’s assassination, Roosevelt actively used the Sherman Anti-trust act to break up holdings of the industrial elite. This progressive era dominated both Democrats and Republicans until conservative Warren Harding’s victory over progressive Ohio Governor James Cox in the 1920 election. Harding’s nomination was pretty much bought and paid for by the industrial elite. Harding’s campaign marked the first time a political campaign relied on commercial advertising consultants and Cox and his running mate, Franklin Delano Roosevelt (FDR) were out spent 4 to 1.
James Cox came from a farming background and lived alongside Kettering and Deeds in Dayton Ohio. Deeds had a close relationship with Durant, selling him ignition systems and self-starters for Cadillac starting in 1911. They were ‘birds of a feather’ and great deal makers. After the great Dayton Flood of 1913, Deeds brought in Arthur Morgan to design protection for the city. Morgan said it could only be addressed in a regional approach. Deeds, Morgan and Dayton attorney, John McMahon wrote legislation to allow for, and give structure to regional conservancy districts. Governor Cox pushed this through the Ohio Assembly and work began on a series of flood protection dams. This conservancy district served as a prototype for the Tennessee Valley Authority (TVA) and Arthur Morgan served as TVA’s first director under FDR. Henry Ford, Edward Deeds, Charles Kettering, James Cox and William Durant all came from farming communities and understood the importance of the agriculture sector. They initially supported the early chemurgy movement to find industrial uses for agriculture products. The defeat of Cox by Harding was bitter for Cox’s supporters and their progressive agendas. This most probably reflected differences in the boardroom of GM where its board of directors broadly represented the industrial elite who supported Harding.
Better Living Through Chemurgy from The Economist
Europe Follows a Different Path
This was not the case in Europe, however, where many countries did not have control of petroleum resources either domestically or from its colonies. Ethanol fuel use was actively being explored in Germany and France in the late 19th century. Many farm applications of the IC engine were operating on ethanol in the 1880’s and 90’s. Between World War I and World War II ethanol blends were extensively used in Europe and elsewhere in the world, especially by countries who wanted domestic fuel resources. See map.
Prohibition was in effect in the US between 1920 and 1933. After prohibition was lifted, a small resurgence of ethanol blending occurred but was met with anti-competitive practices by the Ethyl Corporation. Antitrust suits against the Ethyl Corporation went up the court system resulting in a 1939 U.S. Supreme Court decision against the Ethyl Corporation for violation of the Sherman anti-trust act. While ESSO and GM argued against ethanol blending in the U.S, Cleveland Discol, ESSO’s British subsidiary promoted ethanol blending as being a better fuel. See advertisement below. This blend was sold through 1968.
Progressive Politics Reasserts Itself
The basics of auto engines and fuels remained fairly constant between 1925 and 1975. The rural and farm economy never recovered to a reasonably robust condition with only a small rally during WWII. In 1966 in hearings before the Senate Committee on Public Works, Senator Edward Muskie raised the question of adverse health effects from airborne lead. As new data accumulated on health effects of lead at lower doses, the movement to remove lead from gasoline gained momentum.
The creation of the U.S. Environmental Protection Agency (EPA) in December of 1970 gave a method for regulation of air quality and toxic emissions. The presence of lead in the air was not a concern to the general public, but smog was. Smog could be seen, smelled and tasted but lead was invisible. Science identified four emissions that were significant contributors to smog; NOX, volatile organic compounds (VOC), Carbon Monoxide (CO) and particulate matter. Prevention of their production with petroleum fuels was seen as impossible so a management strategy of filtering and converting them was adopted. The catalytic converter was the main technology for reducing these emissions and smog. In 1970, GM sold its interest in the Ethyl Corporation and then immediately announced that they would begin producing vehicles with catalytic converters that would need unleaded gasoline.
Transition #4 – The Clean Air Act
The Clean Air Act of 1970 required a 75% reduction in emissions from vehicles starting in 1975. For catalytic converters to work, tetraethyl lead could not be added to gasoline as it destroyed the catalytic converter. Oil companies said this could not be done because we needed a higher octane than straight gasoline could provide. Kettering, however, had explored a variety of alternatives.
Kettering had found that adding aromatics was a potential solution. Advanced refining technologies could easily produce aromatics, although at an increased cost. History told us that this additive could also be alcohol. Oil companies also tried for a time to use methyl tert-butyl ether (MTBE) petroleum derived ether that added octane and was also an oxygenate. The Clean Fuels Program began in 1973 which required phasing out use of tetraethyl lead as an octane enhancement. Leaded gasoline was given a 20-year phase out time for use in legacy vehicles and was completely eliminated by 1995. 1975 model year vehicles were required to have catalytic converters. Also, fueling stations were required to have at least one pump with unleaded gasoline. Ethanol was approved as an additive up to 10% in 1979.
The Clean Air Act was amended in 1990. These amendments reduced allowable Reid Vapor Pressure (RVP) of gasoline, added an oxygenate requirement to reduce CO content and required methods to capture and reduce VOCs from escaping the fueling system. This applied to ‘non-attainment’ areas that were primarily on the coasts and big cities. It also gave a 1 pound per square inch (psi) waiver to ethanol blends of 9-10%. This was necessary because of the phobic nature of petroleum and ethanol. The RVP spikes about 1 psi higher that the gasoline blend stock at 10-12% ethanol and then begins to decrease. Limits were also placed on sulfur and aromatics used for octane enhancement using the words “limited to the greatest extent possible.”
E-10 Increasingly Used for Octane Enhancement and as an Oxygenate
In 2003, California banned MTBE, a potential carcinogen, as a gasoline additive and more than half the states followed suit. Soon oil companies abandoned MBTE and accepted the inevitable fact that ethanol is the best and least costly octane enhancement and oxygenate. A 10% ethanol blend was tacitly accepted as the ‘greatest extent possible’ for eliminating aromatics.
With the passing of the Renewable Fuels Standard II (RFS II) in 2007, voluntary inclusion rates of ethanol outpaced mandated levels indicating ethanol’s limited acceptance by the oil industry. For example, 2010 inclusion (demand) rates were almost equal to the 2012 higher mandated level.
Preview of Next Installment
Part 2 of this White Paper will examine the fifth transition involving mid- blends of ethanol (E-30 and E-40) as a way to advance fuels with low health issues (clean), low carbon content and low lifecycle greenhouse gas (GHG) emissions (green) or high fuel efficiency.



Leave a Reply